Diagnosis and Treatment of Mild Traumatic Brain Injury-related Vision Problems for the Primary Care Optometrist ( OTHER)

|
Jeremy Walz, O.D.
|
For a printable copy of this article, click here.
Diagnosis and Treatment of Mild Traumatic Brain Injury-related Vision Problems for the Primary Care Optometrist (OTHER)
|
Introduction Traumatic brain injury (TBI) can happen to anyone and can have life altering consequences. The recent conflicts in Iraq and Afghanistan, as well as the controversy surrounding the National Football League, have significantly increased public awareness of TBI. Given that over 50 percent of the brain is directly or indirectly involved in visual processing it is not surprising that visual symptoms would be amongst the most common long-term sequelae of brain injury.1,2 Historically, many TBI-related vision problems have been overlooked due to a primary emphasis on visual acuity and eye disease. This creates a void in the treatment paradigm that optometrists are uniquely qualified to fill. Many of the conditions that plague these patients are common ones that each of us mastered during optometry school. The purpose of this article is to propose a simple, efficient method to treat many of the common visual dysfunctions following mild TBI in a busy, primary care optometry practice. Background According to the Centers for Disease Control and Prevention, TBI accounted for at least 2.5 million emergency department visits, hospitalizations or deaths in the United States in 2010. This represents a 70 percent increase over the previous decade.3 TBI is a major cause of death and disability in this country, contributing to approximately 30 percent of all injury-related deaths with an estimated direct and indirect cost of $82 billion dollars annually.3 Nearly 2 percent of the population lives with a TBI-related disability.3 Children aged 0 to 4 years, adolescents aged 15 to 19 years, and adults 65 years and older are at the highest risk to sustain a TBI.3 The most common cause of TBI in the civilian population is falls (Figure 1).3 |
.jpg)
Figure 1.
A traumatic brain injury is defined by the Veterans Affairs/Department of Defense (VA/DoD) as a traumatically induced structural injury and/or physiologic disruption of brain function as a result of an external force that is indicated by new onset or worsening of at least one of the following clinical signs immediately following the event: 1) Any period of loss or decreased level of consciousness. 2) Any loss of memory for events immediately before or after the injury 3) Any alteration of mental state (confusion, disorientation, etc.) 4) Neurological deficits (weakness, loss of balance, changes in vision, etc.) or 5) Intracranial lesion.4
Brain injury is caused by two distinct mechanisms. Primary injury occurs at the time of the event and is a direct result of mechanical forces such as a penetrating injury, blast wave, acceleration-deceleration and coup-contrecoup. A coup injury occurs at the point of impact as the head strikes or is struck by an object. The contrecoup injury occurs opposite the site of impact as the brain moves within and strikes the skull. Secondary injury develops hours or days later and is often more severe than the primary injury. It is a biological response to the primary injury such as ischemia, edema, inflammation, neurotoxicity, hemorrhage or increased intracranial pressure. The ultimate level of functional recovery is often dependent on the extent of secondary injury.1,5,6
In contrast to the more localized damage caused by strokes, brain injury due to trauma tends to be more diffuse. The signature of TBI is diffuse axonal injury, which is caused by rapid head movement causing shearing and stretching of axons.6,7 This can disrupt the axonal cytoskeleton leading to inflammation, impaired axonal transportation and global dysfunction.1 Diffuse axonal injury can be difficult to image with computed tomography (CT) and standard magnetic resonance imaging (MRI). Greater than one-third of patients with neuro-ophthalmic deficits following TBI have normal neuroimaging.8 However, the development of new scanning techniques, such as diffusion tensor imaging, that are capable of imaging white matter tracts in the brain can potentially improve our ability to detect damage from TBI.6,7
There is no standardized classification for traumatic brain injury severity. The VA/DoD categorizes TBI as mild, moderate or severe based on the length of lost or altered consciousness, post-traumatic amnesia and the Glasgow coma scale score (Figure 2).4 The Glasgow coma scale is a measure of motor, verbal and eye opening response at 24 hours post injury or later.4 Concussion is a nonspecific term that could refer to any non-penetrating brain injury, however, is more commonly used to refer specifically to a mild TBI. Fortunately, about 75 percent of the TBIs that occur each year fall into the mild category , also commonly called a concussion.3 This classification, however, does not predict the patient’s long-term outcome and many mild TBI suffers experience long-term symptoms.4,9 The sequelae of TBI typically manifest immediately and may resolve within minutes to hours, while others persist for months to years and may even be permanent.5
|
Figure 2. (LOC- Loss of consciousness, AOC- alteration of consciousness, PTA- post-traumatic amnesia, GCS- Glasgow coma scale) |
Visual Dysfunction
Despite 20/20 vision in the vast majority, approximately three quarters of TBI patients have visual symptoms.5,7,10-16 The most common complaints following TBI include: blurred vision, light sensitivity, diplopia, reading difficulty, headaches/eyestrain and visual fatigue.5,10-14,17-19 Visual problems following TBI are often overlooked due to vague complaints and subtle presentations. However, they can have a significantly negative impact on activities of daily living, such as reading, writing, driving, shopping, and dressing and may interfere with the overall rehabilitative progress, employability and quality of life.1,5,6,11,17,18,20-22 Unfortunately, these symptoms do not appear to resolve with the natural recovery process, as demonstrated in a recent study by Capo-Aponte, et al.19
Up to 90 percent of patients will manifest some type of visual dysfunction following TBI. The prevalence of individual conditions varies widely depending on the severity of the injury and the study methods used.1,2,8,11,12,17,23 Both the afferent and efferent visual pathways may be affected. However, vision and visual field loss occur at much higher rates (3.2-39%) in more severely injured patients requiring hospitalization.1,10,13,15,20,24,25 The vergence, accommodative and oculomotor systems are most frequently involved in mild TBI.
Convergence insufficiency is the most common vergence dysfunction after TBI with a prevalence of 23-67 percent, significantly higher than the 3.5-8 percent rate in the general population.1,7,10,13,15,18,20,21 Signs of convergence insufficiency may even arise following sub-concussive head impacts.27 Cohen, et al. found similar rates of convergence insufficiency in both the acute TBI phase and on reevaluation three years later, again indicating that the condition does not spontaneously resolve.26 Accommodative disorders, typically accommodative insufficiency, occur in approximately 10-67 percent of non-presbyopic TBI patients compared to a prevalence of 9 percent in the non-brain injured population.1,7,10,11,15,18,20,21 Oculomotor dysfunction such as saccade and pursuit dysfunction occurs in up to 90 percent of the TBI population versus 20 percent in the general population.1,7,10,15,20,21
Vertical binocular deviations are another significant obstacle for patients following head injury. In a recent study, Capo-Aponte, et al. found a significantly higher prevalence of vertical phoria in a group of blast-injured veterans compared to the control group (55 percent vs. 5 percent).14 Even small vertical phorias can cause significant visual symptoms given our relatively low vertical vergence ranges. Vertical phorias are also frequently associated with vestibular dysfunction, further exacerbating their symptoms.
Frank strabismus occurs in 7-37.5 percent of cases with cranial nerve palsies as a common cause.7,10,15,21 Approximately 6 percent of TBI patients present with cranial nerve 3, 4 or 6 palsies and are typically associated with more severe injuries.1,21 As a result of its anatomy, the fourth nerve is most commonly involved followed by cranial nerve 3.8,13 For this reason as well, a high proportion (32.5 percent) of cranial nerve 4 palsies will be bilateral.8
Light sensitivity is a frequent and vexing complaint, affecting approximately 50 percent of TBI patients.9,10,16 It is most common in the subacute phase; 7-19 days post injury, and often resolves by six months.28 However, in many cases the symptoms persist indefinitely. The exact etiology of photophobia following brain injury is unclear at this time. Goodrich, et al. found that TBI patients concurrently diagnosed with PTSD had a significantly higher rate of subjective light sensitivity compared to patients with TBI alone despite similar objective findings suggesting that photophobia may have a non-organic origin.12 However, the relatively recent discovery of intrinsically photosensitive retinal ganglion cells and their direct connection to pain sensitive nuclei in the thalamus have helped to shed some light on a potential underlying etiology.28
Assessment
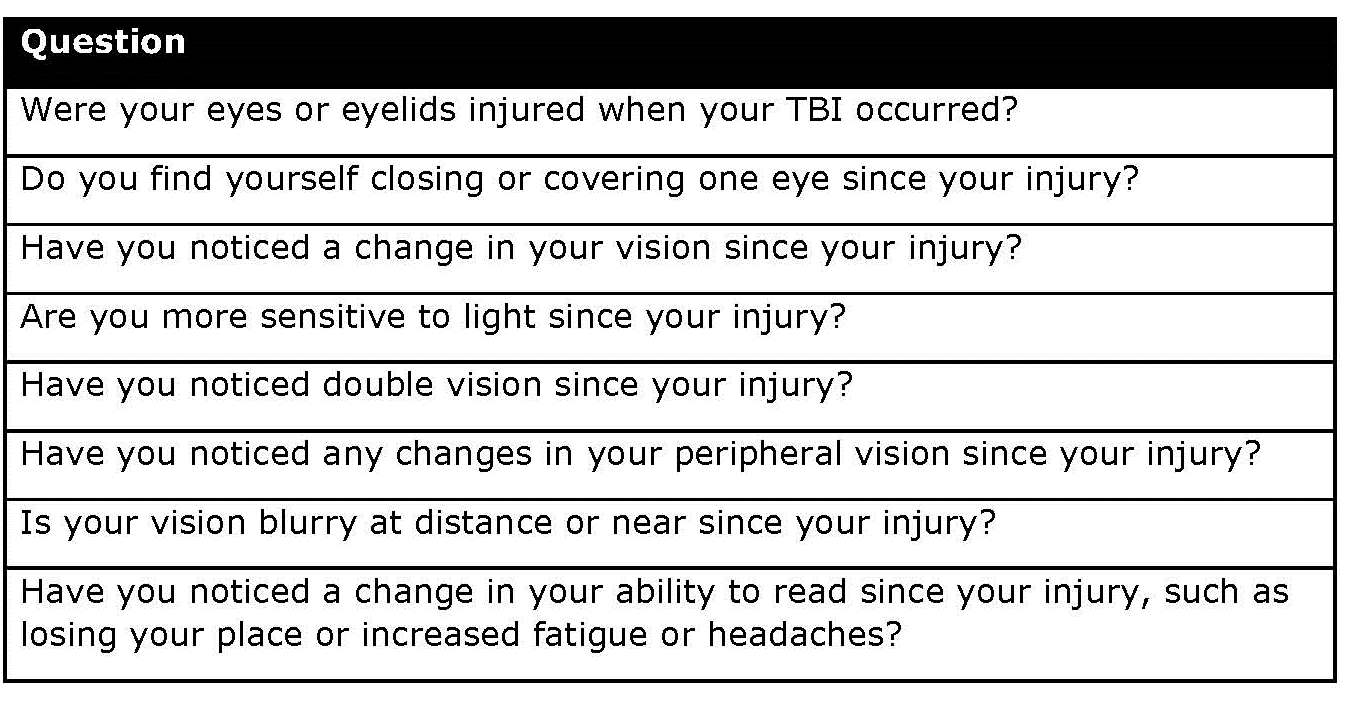
As with any condition, a thorough history is vital in cases of TBI. Patients should be questioned in detail about changes in their vision since the injury, particularly those related to reading. A written questionnaire, such as the Convergence Insufficiency Symptom Survey, can be useful to uncover often- overlooked symptoms and save chair time. Figure 3 contains examples of recommended TBI vision history questions adapted from a recent Delphi study by Goodrich, et al.29
|
Figure 3. |
Diagnosis of the visual conditions associated with TBI often requires more in-depth binocular vision and accommodative testing than can be performed during a routine comprehensive exam. However, a gross visual screening can provide helpful information without significantly increasing chair time. In addition to a routine cover test and careful monitoring of pursuit accuracy during extra-ocular motility testing, this screening should include near point of convergence, monocular amplitudes of accommodation and saccadic testing. This ought to add no more than a few minutes to the overall exam time. If the patient is symptomatic or fails any part of the initial screening, a follow-up visit can be scheduled for more detailed testing including, at minimum, lateral and vertical phorias, fusional vergence ranges and monocular accommodative facility. Determination of the accommodative convergence to accommodation (AC/A) ratio is also crucial to obtaining the correct diagnosis and guiding treatment.
As a general rule, out of phoropter testing is preferred as it is often easier on the patient and may provide more accurate results. Maddox rod testing can be helpful to detect and measure small vertical phorias or for cyclovertical deviations. Eye alignment cards, such as the Modified Thorington, are a quick and easy way to subjectively measure phorias and assess AC/A ratio. The King Devick and Developmental Eye Movement (DEM) tests can also be useful to further assess oculomotor problems. Once the data is collected an integrative analysis, considering the direction and magnitude of the deviation at distance versus near along with the AC/A ratio, will help to identify the correct underlying dysfunction (Figure 4).
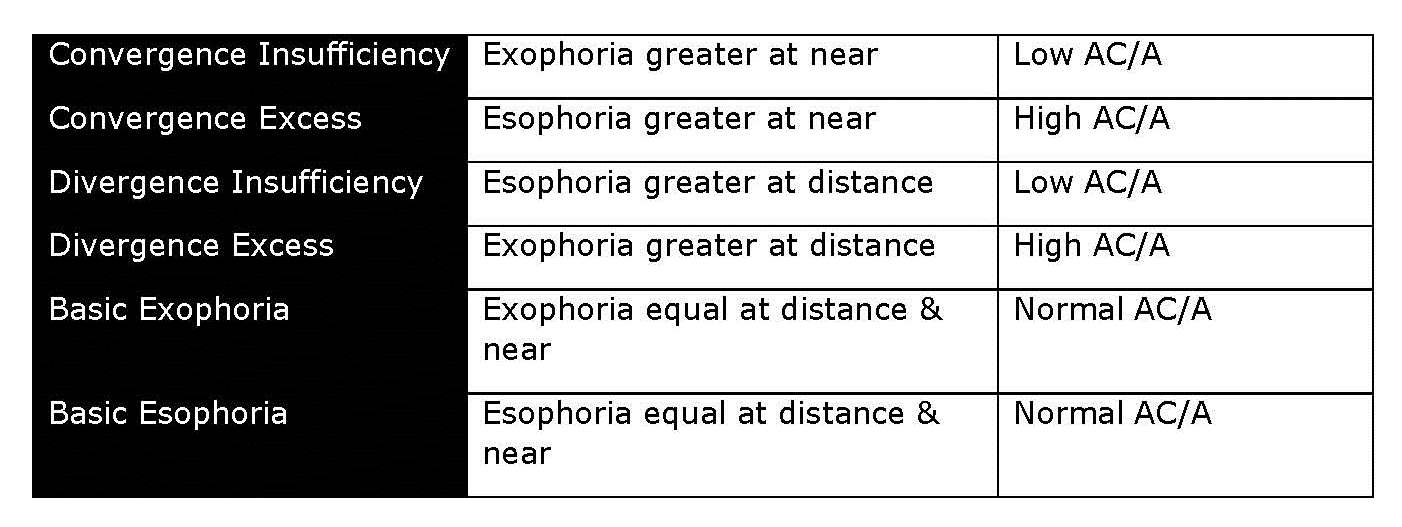 |
Figure 4. – Duane’s Classification
Treatment
There are a limited number of treatment options for TBI-related visual dysfunction. It is helpful to develop a habit of considering all available options in a sequential manner for every patient. While every alternative should not be prescribed, thinking through each will ensure that no potential solution is overlooked and will improve the likelihood of success.
Correction of significant refractive error should always be the primary treatment option. Uncorrected refractive error may cause under or over accommodation leading to accommodative dysfunction, increased fusional vergence demand and decreased fusional ability. It is typically recommended to prescribe maximum plus for esodeviations and minimum plus for exodeviations. The definition of significant refractive error for symptomatic patients diagnosed with TBI should be slightly less than the general population (Figure 4).20 It is also recommended, at this point, to prescribe vertical prism, equal to the full deviation, for all symptomatic patients with any amount of vertical phoria, particularly those with vestibular symptoms. Frequently, correction of even a small vertical deviation can normalize binocular functioning to the point that other oculomotor conditions resolve spontaneously.20 Patients should wear the correction for 4 to 6 weeks and return for follow-up binocular vision testing.
.jpg) |
Figure 5.
If symptoms persist, consider if additional lens power at distance or near would be beneficial. AC/A ratio is the key to determining the usefulness of additional lenses. In addition to accommodative dysfunction, convergence excess and basic esophoria respond well to added plus at near. Increased minus in the distance can be helpful for divergence excess, considering accommodative function is normal. The amount of additional power needed can be ascertained by monocular estimation method (MEM) retinoscopy or NRA/PRA testing. For TBI patients requiring a near add, it is recommended that separate pairs of glasses be prescribed at distance and near in lieu of a bifocal or progressive design due to the increased prevalence of vestibular dysfunction and mobility problems in this population.
Horizontal prisms are next in the management sequence. Prisms are most effective as standalone treatments for divergence insufficiency. Prisms may also be prescribed in cases of convergence insufficiency when the patient is unmotivated or unable to participate in vision therapy due to concurrent cognitive or physical disorders. Prisms have been shown to be ineffective in children with convergence insufficiency; however, they have not been thoroughly studied in a TBI population. The necessary amount of horizontal prism can be determined based on the minimum amount required to provide subjective fusion using red/green glasses with a Worth 4 Dot or penlight or by Sheard’s criterion. Sheard’s criterion states that for a patient to be visually comfortable the compensating fusional vergence should be twice the phoria.20
Prism may also be useful as an adjunctive treatment during or after vision therapy. While vision therapy has been proven effective in the treatment of convergence insufficiency in both children and adults, it has not been studied in a randomized controlled manner in a TBI population. However, multiple retrospective studies and case studies/series do suggest that it may be effectual.20,30-36 Vision therapy can also be helpful for pursuit and saccadic dysfunction. The efficacy of vision therapy may be impaired by other factors associated with TBI, such as cognitive or perceptual dysfunction and visual field defects, as reported by Scheiman and Galloway.20,30 Therefore, these factors should be considered when evaluating the suitability of vision therapy. Optometrists who are not comfortable or have not been trained in prescribing and managing a vision therapy protocol should be apt to refer patients who could potentially benefit to a fellow OD who does provide these services. In cases where in-office vision therapy is not feasible, due to physical or travel constraints, computerized home therapy programs are available.
Surgical management of paretic or incomitant strabismus secondary to cranial nerve or supranuclear palsies should be deferred and patients should be monitored for a minimum of six months until the deviation stabilizes. These deviations can spontaneously resolve during the natural recovery process, although the prognosis is variable.13 During this period, alternating occlusion can be a helpful short-term solution to relieve diplopia. Fresnel prism can also be useful, but must be changed frequently as the deviation subsides.
Occasionally, despite our best efforts, some patients are unable to achieve single vision, due to a cyclovertical deviation or significant noncomitancy. In these cases occlusion may be the only option. When prescribing occlusion it is important to balance the level of occlusion necessary with the cosmetic effect of the proposed treatment. Many TBI patients are young and unaccepting of the standard ”pirate patch.” Bangerter foils and occlusive contact lenses can be very helpful in this population. In cases where reading symptoms are due to saccadic dysfunction, a typoscope is a simple treatment option. A typoscope is an opaque device with an aperture to isolate the text of regard while obscuring non-pertinent text.
Treatment of photophobia can be challenging. Patients should be discouraged from wearing extremely dark tints, as this can lead to chronic dark adaptation of the retina thereby worsening the patient’s symptoms. Amber tinted ”blue blocker” lenses may be most beneficial at reducing symptoms as TBI patients may be more sensitive to wavelengths in the blue portion of the spectrum.37 Artificial pupil contact lenses can be helpful for multiple reasons: not only do they reduce the amount of light reaching the peripheral retina without dark adapting the macula, they may also contribute to trigeminal desensitization.37 Topical medications such as brimonidine and pilocarpine may also reduce symptoms by inducing miosis, however, the side effects of these medications may impede their usefulness. Lastly, dry eye syndrome should be treated aggressively in patients with concurrent photophobia as trigeminal irritation may exacerbate symptoms.
.jpg) |
Figure 6.
Conclusion
Traumatic brain injury can have a profound impact on visual function despite normal visual acuity and ocular health. The vergence, accommodative and oculomotor systems are uniquely susceptible. Disruption of any one of these systems can cause impaired or inefficient visual function and reduced quality of life. Reading is one area of particular difficulty for TBI patients. We, even as eye care providers, often take for granted the complex task of reading which requires accurate integration of vergence, version and accommodation in addition to its attention and cognitive demands.14 Treatment of visual disorders following TBI can be challenging due to concurrent physical, cognitive, behavioral, psychological and sensory dysfunction.6,20 However, we all have the skills and the tools necessary to manage them. If we just take the time to listen and overcome our fear of the unknown, we can potentially have a profound impact on someone’s quality of life.
Reference
1. Alvarez T, et al. Concurrent vision dysfunctions in convergence insufficiency with traumatic brain injury. Optom Vis Sci 2012; 12: 89-112.
2. Dougherty A, et al. Visual dysfunction following blast-related traumatic brain injury from the battlefield. Brain Injury 2011: 25(1): 8-13.
3. Centers for Disease Control and Prevention (2015). Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. Atlanta, GA.
4. Department of Veterans Affairs, Department of Defense (2009). VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. The Management of Concussion/mTBI Working Group. Version 1.0.
5. Kelts EA. Traumatic brain injury and visual dysfunction: a limited overview. NeuroRehabilitation 2010; 27: 223-229.
6. Thiagarajan P, Ciuffreda KJ & Ludlam DP. Vergence dysfunction in mild traumatic brain injury (mTBI): a review. Ophthalmic Physiol Opt 2011; 31: 456–468.
7. Cockerham G, et al. Eye and visual function in traumatic brain injury. J Rehabil Res Dev 2009; 46: 811-818.
8. Van Stavern G, et al. Neuro-ophthalmic manifestations of head trauma. J Neuro-Ophth 2001; 21(2): 112-117.
9. O’Neil ME, et al. Visual Dysfunction in Patients with Traumatic Brain Injury: A Systematic Review. VA Evidence-based Synthesis Program Project #05-225; 2014.
10. Goodrich G, et al. Mechanisms of TBI and visual consequences in military and veteran populations. Optom Vis Sci 2013; 90: 105-112.
11. Goodrich G, et al. Visual function in patients of a polytrauma rehabilitation center: a descriptive study. J Rehabil Res Dev 2007; 44: 929-936.
12. Goodrich G, et al. Visual function, traumatic brain injury, and posttraumatic stress disorder. J Rehabil Res Dev 2014; 51: 547-558.
13. Sabates N, et al. Neuro-ophthalmologic findings in closed head trauma. J Clin Neuro-ophthalmol 1991; 11(4): 273-277.
14. Capo’-Aponte J, et al. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Military Medicine 2012; 177(7): 804-813.
15. Brahm K et al. Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury. Optom Vis Sci 2009; 86: 817-825.
16. Stelmack J, et al. Visual function in patients followed at a Veterans Affairs polytrauma network site: an electronic medical record review. Optometry 2009; 80: 419-424.
17. Kapoor N, Ciuffreda K. Vision disturbances following traumatic brain injury. Curr Treat Options Neurol 2002; 4: 271-280.
18. Greenwald B, et al. Visual impairments in the first year after traumatic brain injury. Brain Injury 2012, 26(11): 1338-1359.
19. Capo’-Aponte J, et al. Visual Dysfunctions at different stages after blast and non-blast mild traumatic brain injury. Optom Vis Sci 2016; 93: [Epub ahead of print].
20. Scheiman M. Understanding and managing vision disorders after traumatic brain injury: A guide for military optometrists. Department of Veterans Affairs/Department of Defense; 2011.
21. Ciuffreda K, et al. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry 2007; 78: 155-161.
22. Lemke S, et al. Visual quality of life in veterans with blast-induced traumatic brain injury. JAMA Ophthalmol 2013; 131(12): 1602-1609.
23. Hunt A, et al. Oculomotor based vision assessment in mild traumatic brain injury: a systematic review. J Head Trauma Rehabil 2016; 31(4): 252-261.
24. Suchoff I, et al. The frequency of occurrence, types, and characteristics of visual field defects in acquired brain injury: a retrospective analysis. Optometry 2008; 79: 259-265.
25. Lemke S, et al. Automated perimetry and visual dysfunction in blast-related traumatic brain injury. Ophthalmology 2016; 123: 415-424.
26. Cohen M, et al. Convergence insufficiency in brain-injured patients. Brain Injury 1989; 3: 187-191.
27. Kawata K, et al. Association of football subconcussive head impacts with ocular near point of convergence. JAMA Ophthalmol 2016; 134(7): 763-769.
28. Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol 2012; 32(1): 68-81.
29. Goodrich G, et al. Development of a mild traumatic brain injury-specific vision screening protocol: A Delphi study. J Rehabil Res Dev 2013; 50: 757-768.
30. Scheiman M, Gallaway MF. Vision rehabilitation to treat binocular vision disorders after acquired brain injury: Factors affecting prognosis. In: Suchoff I, Cuiffreda, KJ, Kapoor N, ed. Visual and Vestibular Consequences of Acquired Brain Injury. Santa Ana: Optometric Extension Program; 2001.
31. Ciuffreda K, et al. Vision therapy for oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry 2008; 79: 18-22.
32. Kapoor N, et al. Oculomotor rehabilitation in acquired brain injury: a case series. Arch Phys Med Rehabil. 2004; 85(10): 1667-1678.
33. Ciuffreda K, et al. Oculomotor rehabilitation in traumatic brain-injured patients. J Beh Optom 1996; 7: 31-37.
34. Cohen AH. Optometric management of binocular dysfunctions secondary to head trauma: case reports. J Am Optom Assoc 1992; 63: 569-75.
35. Hellerstein LF, Freed S. Rehabilitative optometric management of a traumatic brain injury patient. J Beh Optom 1994; 5: 143-48.
36. Thiagarajan P, et al. Oculomotor neurorehabilitation for reading in mild traumatic brain injury (mTBI): an integrative approach. NeuroRehabilitation. 2014; 34: 129–146.
37. Nosedo R, et al. A neural mechanism for exacerbation of headache by light. Nature Neurosci 2010; 13(2): 239-246.
| Click HERE to take the test |
1.png)

1.png)


.png)




.png)
.png)
.png)
.jpg)
.png)

.jpg)
.png)
.png)
.png)
.png)
.png)

.png)

.png)
.png)
